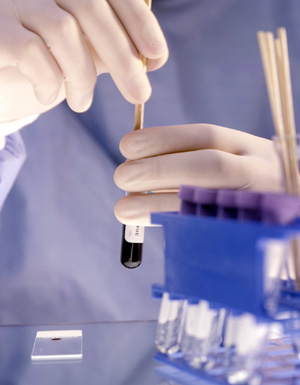
Clinical Research Trials
What is Clinical Trials?
Clinical Trials are research studies designed to test the safety and effectiveness of new medications and treatments. Clinical trials are required by the Food and Drug Administration before a drug and/or treatment plan can be released to the public market.
Can I participate in a research study?
Research studies in our office are open to all our patients who meet the standard requirements, set forth by the company sponsoring the study. A licensed member of our team regularly views the patient files and may contact you if you qualify.
How do I participate?
We are participating in and actively recruiting for patients to participate in clinical trials for several types of disease.
If you are interested to participate in trials please contact our study coordinator, at our office, Renee Williams, RN at (951) 781-7700.

 Riverside6180 Brockton Avenue, Riverside, CA 92506
Riverside6180 Brockton Avenue, Riverside, CA 92506 La Sierra4244 Riverwalk Parkway, Riverside, CA 92505
La Sierra4244 Riverwalk Parkway, Riverside, CA 92505